Abstract
Introduction: CD19, a B-cell surface antigen, is overexpressed in neoplastic lymphoid cells. ADCT-402 (loncastuximab tesirine; Lonca-T) is an antibody drug conjugate comprising a humanized monoclonal antibody directed against human CD19 conjugated through a pyrrolobenzodiazepine dimer toxin. This first-in-human clinical study evaluated the safety and efficacy of Lonca-T in patients (pts) with relapsed/refractory (R/R) B-cell lineage non-Hodgkin lymphoma. In the United States, follicular lymphoma (FL) is the most common indolent lymphoma and constitutes approximately 20% of all lymphomas, while mantle cell lymphoma (MCL) is rare and represents approximately 5% of all lymphomas. Here we present interim results in a subgroup of pts with FL and MCL; interim safety and efficacy of Lonca-T in pts with diffuse large B-cell lymphoma is presented in a separate abstract.
Methods: Pts (≥18 years of age) with R/R FL and MCL who have failed or are intolerant to established therapies, or have no other treatment options available, were enrolled in this Phase 1, multicenter, open-label, and single-arm study including dose-escalation and dose-expansion parts. The primary objectives are to evaluate the safety and tolerability of Lonca-T, and determine the recommended dose(s) to use for expansion cohorts. The secondary objectives are to evaluate the clinical activity (measured by overall response rate [ORR], duration of response [DoR], progression-free survival [PFS] and overall survival), pharmacokinetics, pharmacodynamics, and anti-drug antibody activity. Pts received 1-hour intravenous infusions of Lonca-T every 3 weeks (Q3W; 1 cycle), with a 3+3 dose-escalation study design. No intra-pt dose-escalation is allowed.
Results: As of June 20, 2018, 15 pts with FL (12 male, 3 female) and 15 with MCL (4 male, 11 female) had been enrolled in the study. The median ages of pts with FL and MCL were 58 years [range 40-75] and 64 years [range 51-87], respectively. Pts with FL had received a median 4 previous therapies (range 1-9), and those with MCL had received a median 4 prior therapies (range 1-13; Table), including prior ibrutinib therapy in 2/15 (13.3%) and 10/15 (66.7%) pts, respectively. Pts received doses of Lonca-T ranging from 15 to 200 µg/kg Q3W (FL, median cycles: 3 [range 2-11] and MCL, median cycles: 2 [range 1-11]).
Of all pts with FL or MCL, treatment-emergent adverse events (TEAEs) were reported in 29/30 (96.7%) pts, and grade ≥3 TEAEs in 20 (66.7%) pts. The most common all-grade TEAEs (≥20% pts), regardless of relationship to study treatment, were fatigue (13 [43.3%]), increased gamma-glutamyltransferase (GGT) (13 [43.3%]), anemia (10 [33.3%]), myalgia (9 [30.0%]), increased alkaline phosphatase (8 [26.7%]), dyspnea (8 [26.7%]), nausea (8 [26.7%]), peripheral edema (8 [26.7%]), pleural effusion (8 [26.7%]), abdominal pain (7 [23.3%]), erythema (7 [23.3%]), decreased neutrophil count (7 [23.3%]), increased alanine transferase (6 [20.0]), increased aspartate aminotransferase (6 [20]), constipation (6 [20.0]), decreased appetite (6 [20.0]), and headache (6 [20.0]). The most common grade ≥3 TEAEs (>10% pts) were increased GGT (8 [26.7%]), decreased neutrophil count (6 [20.0%]), and anemia (4 [13.3%] pts).
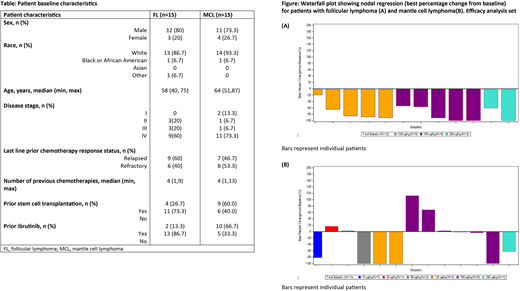
The figure depicts tumor response data for pts with FL and MCL. For the 15 evaluable pts with FL, the ORR was 80% (12/15 pts), comprising 8/15 (53.3%) complete responses (CRs) and 4/15 (26.7%) partial responses (PRs). The median DoR and PFS (responders and non-responders) were not reached in pts with FL after a median follow-up time of 7.56 months. Out of 15 evaluable pts with MCL, the ORR was 7/15 (46.7%), comprising 4/15 (26.7%) CRs and 3/15 (20.0%) PRs. In pts with MCL, median DoR was 5.3 months and PFS was 4.8 months after a median follow-up time of 5.78 months.
Conclusions: In this Phase 1 study, Lonca-T has demonstrated encouraging single-agent antitumor activity and manageable toxicity in pts with R/R FL and MCL.
Study sponsored by ADC Therapeutics. http://clinicaltrials.gov/show/NCT02669017.
Caimi:Genentech: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Kite Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau. Kahl:Genentech: Consultancy; Seattle Genetics: Consultancy; ADC Therapeutics: Research Funding. Hamadani:Merck: Research Funding; ADC Therapeutics: Research Funding; Sanofi Genzyme: Research Funding, Speakers Bureau; Janssen: Consultancy; Takeda: Research Funding; Cellerant: Consultancy; Celgene Corporation: Consultancy; MedImmune: Consultancy, Research Funding; Ostuka: Research Funding. Carlo-Stella:Rhizen Pharmaceuticals: Research Funding; Sanofi: Consultancy; Boehringher Ingelheim Italia: Consultancy; MSD Italia: Speakers Bureau; Genenta Science: Speakers Bureau; AstraZeneca: Speakers Bureau; Amgen: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau; Janssen: Speakers Bureau; ADC Therapeutics: Research Funding, Speakers Bureau. He:ADC Therapeutics: Employment, Equity Ownership. Ungar:ADC Therapeutics: Employment, Equity Ownership. Feingold:ADC Therapeutics: Employment, Equity Ownership. Ardeshna:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Takeda: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Radford:ADC Therapeutics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Speakers Bureau; Celgene: Research Funding; BMS: Consultancy, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Equity Ownership; GlaxoSmithKline: Equity Ownership; Novartis: Consultancy, Speakers Bureau; Pfizer: Research Funding. Solh:Celgene: Speakers Bureau; ADC Therapeutics: Research Funding; Amgen: Speakers Bureau. Heffner:Kite Pharmaceuticals: Research Funding; ADC Therapeutics: Research Funding; Genentech: Research Funding; Pharmacyclics: Research Funding. O'Connor:ADC Therapeutics: Research Funding; Celgene: Research Funding; Seattle Genetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal